Seeking Your Comments on Regulation to Change the Program

The BioPreferred Program was established by Congress through the 2002 Farm Bill and was subsequently expanded by the 2008 and 2014 Farm Bills. After the 2002 Farm Bill was authorized, USDA developed initial guidelines to initiate the program, including identifying product categories within which biobased products would be afforded procurement preference by Federal agencies and their contractors.
The 2008 and 2014 Farm Bills directed USDA to amend and expand the program's original guidelines. Some of the major revisions included designating product categories for finished products that are made from designated intermediate ingredients or feedstocks, and allowing products made from forestry and other traditional biobased materials to participate in the Program. The process of developing regulations that appear in the CFR (the Code of Federal Regulations, the "rulemaking" process) involves proposing regulations in the Federal Register, allowing a 60-day public comment period for the proposed regulations, and publishing a final rule in the Federal Register.

Currently, the Program has 97 product categories that have been designated for mandatory Federal purchasing and that appear in the CFR. The Program has proposed and finalized these product categories over the years in a series of 10 rulemaking actions. These rulemaking actions have been referred to as "rounds", and each of the 10 "rounds" designated about 10 new product categories. As directed by the 2014 Farm Bill in February 2014, USDA has proposed a "Round 11" rulemaking that would designate the following 12 intermediate/upstream material product categories: Plastic Resins; Chemicals; Paint and Coating Components; Textile Processing Materials; Foams; Fibers and Fabrics; Lubricant Components; Binders; Cleaner Components; Personal Care Product Components; Oils, Fats, and Waxes; and Rubber Materials.
USDA is also proposing minimum biobased contents for each of these product categories. The public comment period for this proposed action will close on April 13, 2017. Although the Federal government does not typically purchase large quantities of intermediate ingredients and feedstock materials, designating such materials is a means to identify and include finished products made from such designated materials in the Federal preferred procurement program. In a future rulemaking action, USDA will propose designating product categories comprised of finished products made from the intermediate ingredients that have been designated. We invite you to participate in the current regulatory process by reviewing and commenting on the proposed regulations. To begin, please click here and follow the instructions as published in the Federal Register.
Laboratory Options
The following laboratories have been accepted by SEI to conduct testing for the USDA BioPreferred Program's voluntary biobased product certification and labeling initiative in accordance with ASTM D6866-16:
|
Beta Analytic Inc.
|

|
|
Center for Applied Isotope Studies at The University of Georgia
|

|
Prices and processing time vary by lab. For further information, please visit SEI's website.
Featured Frequent Asked Question(FAQ)
What should I do if the name of my company or product changes?
Any company or product name changes must be documented in the BioPreferred Program's online system. Please follow the Program's product and company name change procedures, which can be downloaded from the FAQs page.
What is the difference between products with variations and product families?
Standalone products are products that do not share a formulation with any other products in your company account. If the product comes packaged in multiple sizes (for example, 12 oz. and 16 oz.) then the product varies in size, and each size should be listed as a variation. Products can vary in several aspects, including size, color, and scent. Note that some variations can cause slight changes in the biobased content of a product, for example, adding an essential oil as fragrance to a liquid may slightly increase the biobased content. All variations must share the same or similar formulations, and the biobased content must be within ±3% of the product variation that is tested. We recommend testing the product variation with the lowest biobased content to represent all variations of a product. Variations of the same product (different sizes, colors, or scents) are not considered to be separate products and should not be added to a product family together.
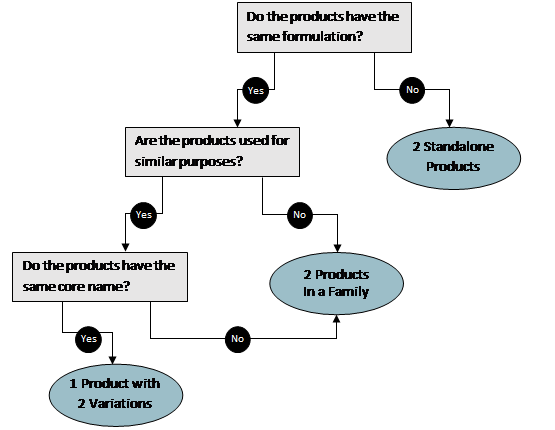
Product families are for products in the same company account that have similar formulations (their biobased contents are within ±3% of one another) but different product names. The biobased content of only one product per family must be tested. For example, if a formulation has multiple uses as a lip balm and a grease, each use could be added to the same product family under a different product name that depended on how the product is marketed. If the grease product was sold in multiple sizes, those sizes can still be added as variations to the product before it is added to the product family with the lip balm. The flow chart below may help you understand whether to register your product as a standalone product or to put it in a product family.
USDA Certified Biobased Products (Packages) - Audit Stage III - Status
BioPreferred's biobased certification and labeling program began in February 2011. Nearly 1,000 (933) of the current ~3,000 certified and labeled products were certified in the first year. Since 2012, the Program has implemented Stage I and II audits to update the certified biobased product content, and information about a participant/company. Product audit history and a summary of the staged approach are available on the BioPreferred web site (no longer active).
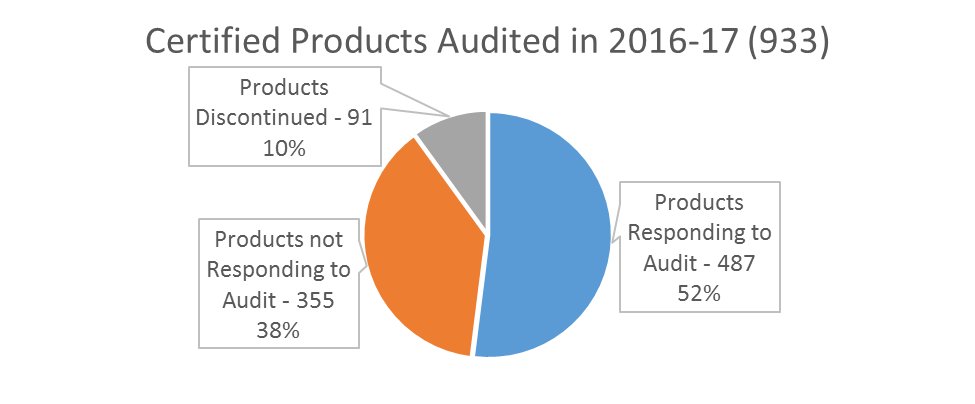
The current audit strategy for certified products, called Stage III, began in fall 2016. As of March 2017, 92% of companies that responded to the audit fall within following three categories:
- Biobased content result (%) from the audit testing is the same as the original biobased content (%);
- Biobased content result (%) from the audit testing is within +/- 3% of the original biobased content (%) and still above the applicable product category minimum biobased content; and
- Biobased content result (%) from the audit testing is more than 3% higher than the original biobased content (%).
The program's product audit strategy will begin again once Stage III concludes at the end of June 2017. A task group has been set up to streamline the audit process to make it, going forward, as efficient and participant friendly as possible.
biopreferred.gov - Our Feature-Rich -- and Secure -- Program Portal
The BioPreferred program contracts with USDA's Enterprise Application Services (EAS) for the vital work of developing and maintaining our web applications and automation software. EAS works closely with the USDA governance boards to ensure that our web applications remain secure and reliable. One of the more visible governance groups can be viewed at DigitalGov.gov. This oversight group enforces many of the security policies and has created a public website that displays the level at which different programs are adhering to these policies.
BioPreferred has earned the highest possible rating of A+ from DigitalGov.gov and were praised on achieving this ahead of the Federal deadline. In addition, we have recently implemented all of the requested DigitalGov analytics trackers. These analytics will enable BioPreferred to put appropriate resources into the features of the web site that field the most traffic or have greater resource needs. We are committed to providing you the needed functionality paired with security measures so our web site may best serve your needs.

 305-662-7760
305-662-7760